Kidney Organoids for SARS-CoV-2 Research
Why Are Kidney Organoids a Relevant Model?
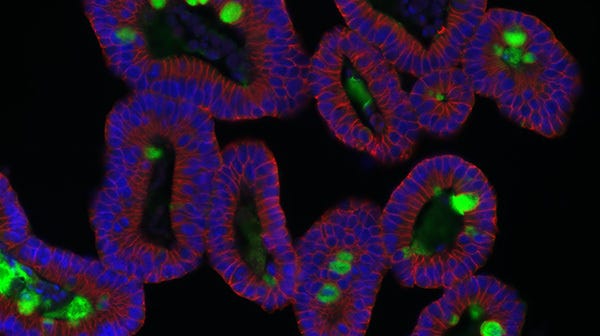
COVID-19 disease is characterized by multi-organ failure, indicating possible viral infection of a wide variety of tissues. Kidney organoids have been shown to express the coronavirus receptor, angiotensin I converting enzyme 2 (ACE2), and may therefore aid researchers in studying the systemic impacts of the disease.
Outside of the lung, the role of epithelial tissues in the pathology and transmission of SARS-CoV-2 is still poorly understood. Previous studies1 of related SARS and MERS-CoV outbreaks reported that acute kidney injury developed in 5 - 15% cases and carried a high mortality rate. The precise role of the kidney in COVID-19 pathology remains to be elucidated and significant insight can be gained using advanced in vitro culture systems that allow for organ-specific tissue modeling.
Key Publication
Monteil V et al. (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. J Cell. Apr 13 Epub ahead of print, DOI: 10.1016/j.cell.2020.04.004.
- See recent news related to this publication >
To determine whether SARS-CoV-2 could directly infect human tubular kidney cells, Monteil and colleagues generated kidney organoids that were then infected with SARS-CoV-2. They found that SARS-CoV-2 replicated in kidney organoids and produced infectious progeny virions. It was also found that human recombinant soluble ACE2 significantly reduced SARS-CoV-2 infection of human kidney organoids.
Kidney Organoids for COVID-19 Research
Although adult kidneys are capable of repair in response to injury or damage, nephrogenesis is complete by week 38 of fetal development in humans, and the identification of an adult kidney stem cell has so far been elusive.2 This has made the study of human kidneys difficult due to the lack of a good in vitro culture system and the limited relevance of animal model systems to the human kidney.
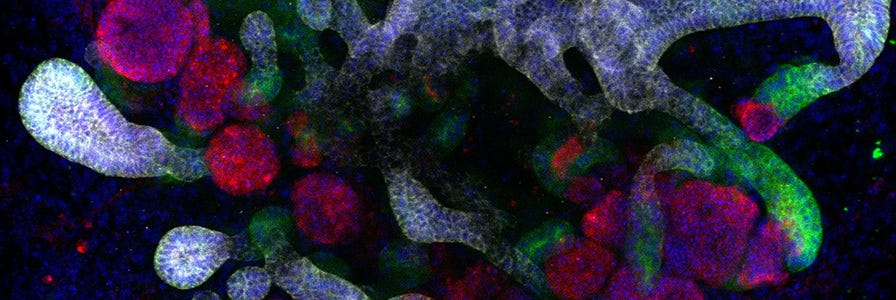
Kidney organoids derived from human pluripotent stem cells (hPSCs) model the structure, as well as the cellular and molecular makeup of the developing human kidney (Figure 1). Like the in vivo kidney, kidney organoids express ACE2, which is now widely recognized as the binding receptor for SARS-CoV-2, and TMRPSS2, which activates the binding of viral proteins to ACE2.3 Furthermore, SARS-CoV-2 infection and replication to form infectious progeny has been demonstrated in hPSC-derived kidney organoids.3
During differentiation from hPSCs, kidney organoids form convoluted tubular structures that resemble the structure and segmentation of the developing human nephron (Figure 1). These organoids contain endothelial cells, mesenchymal cells, podocytes, and epithelial cells of the proximal and distal tubules.4 As organoids retain the genotype and phenotype of their parent cells, in vitro models of kidney disease can be created by reprogramming patient-derived cells or introducing specific mutations through CRISPR-Cas9 gene editing of hPSCs prior to differentiation. Additionally, kidney organoids can be grown in 96- and 384-well formats, enabling high-throughput screening of potential therapeutics for efficacy and nephrotoxicity.
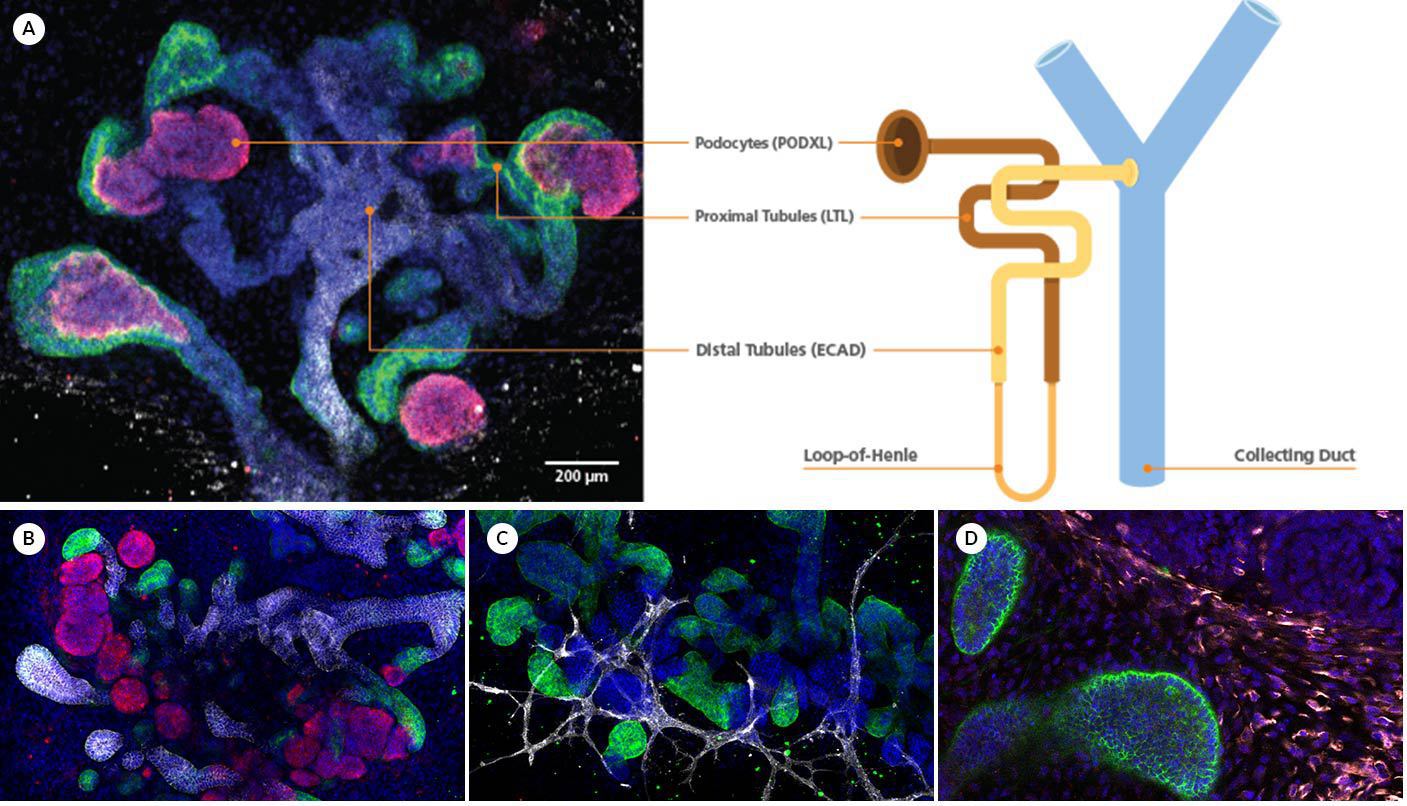
Figure 1. Kidney Organoids Form Convoluted Tubular Structures with Typical Nephron-Like Segmentation
(A) During differentiation using STEMdiff™ Kidney Organoid Kit, kidney organoids form convoluted tubular structures that resemble the structure and segmentation of the developing nephron. These organoids express markers of the (B) renal epithelium, including podocalyxin (PODXL, red), lotus tetragonolobus lectin (LTL, green), and E-cadherin (ECAD, white) as well as markers of the (C) endothelium (platelet endothelial cell adhesion molecule, CD31, white); and (D) mesenchyme (vimentin, VIM, white; Meis homeobox family, MEIS1/2/3, red).
How Are Kidney Organoids Grown?
Like other organoid systems, kidney organoids are generated by using extrinsic signals provided by the cell culture conditions to trigger the biological differentiation pathways that lead to development of the kidney in vivo. hPSCs are cultured under conditions that trigger the early renal differentiation pathway to late primitive streak and allow cells to progress through the subsequent intermediate mesoderm and metanephric mesenchyme developmental stages before forming tubular kidney organoids (Figure 2). The resultant organoids display remarkable segmental patterning and incorporation of a wide range of renal cell types. This recapitulation of the characteristics observed in vivo is characteristic of organoid systems, which leverage the robustness of cell-intrinsic biological programming to generate organotypic models.
The STEMdiff™ Kidney Organoid Kit enables the robust growth of tubular kidney organoids from hPSCs in 21 days (Figure 2). Kidney organoids grown using STEMdiff™ Kidney Organoid Kit resemble the developing kidney and display markers of podocytes, proximal tubules, distal tubules, the endothelium, and mesenchyme (Figure 1). These organoids are suitable for a wide range of experimental contexts, including infectious disease research, developmental and cell biology, disease modeling, drug screening, nephrotoxicity assessment, and cell therapy research.
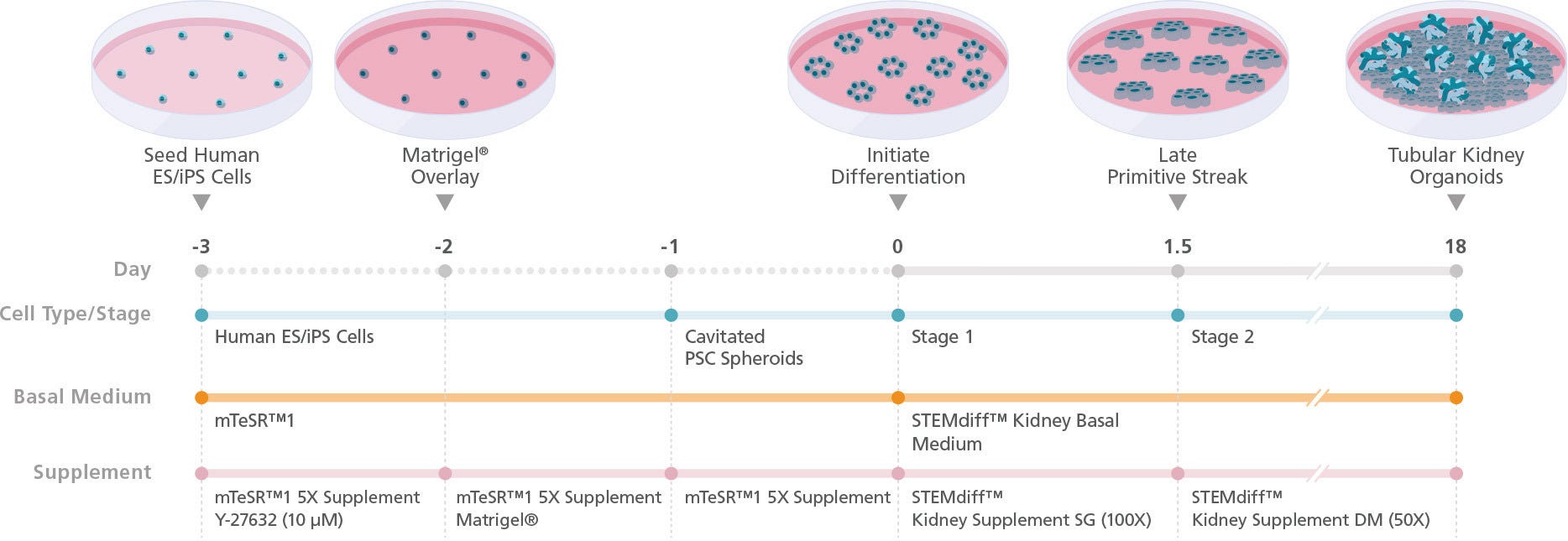
Figure 2. Schematic for Differentiation from hPSCs to Human Kidney Organoids with STEMdiff™ Kidney Organoid Kit
hPSC cultures progress through a simple two-stage process to generate kidney organoids. hPSCs are first plated and overlaid with Corning® Matrigel® to form cavitated spheroids. On the following day (Day 0), differentiation of cavitated PSC spheroids is initiated by changing medium from mTeSR™1 to STEMdiff™ Kidney Organoid Kit. During the next 18 days, the cells are directed through stages of the late primitive streak, intermediate mesoderm, and metanephric mesoderm to give rise to kidney organoids that are composed of podocytes, proximal and distal tubules, and an associated endothelium and mesenchyme.
Products for COVID-19 Research
STEMdiff™ Kidney Organoid Kit
Model the developing nephron and associated endothelium and mesenchyme using kidney organoids derived from hPSCs with a simple two-stage protocol.
Tools for Other hPSC-Derived Model Systems
Relevant, human model systems are a critical component to the global COVID-19 research effort. In vitro cultures generated by directed differentiation of hPSCs are used widely to model a multitude of biological systems.
Stabilized feeder-free maintenance medium for human ES and iPS cells
Related Resources
Nature Research Roundtable: Organoids
Learn key insights from the discussions and watch the presentations from our Nature Research Roundtable on organoids.
Re-Creating Disease with Kidney Organoids and CRISPR
Dr. Benjamin Freedman provides an overview of re-creating disease using kidney organoids and CRISPR-Cas9 genome editing.
Organoid E-Book
Read the Wiley "Essential Knowledge Briefing" on the evolution and application of organoid research.
References
- Naicker S et al. (2020) The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 97(5): 824‐828.
- Wu H et al. (2018). Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. 23(6): 869–881.
- Monteil V et al. (2020). Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 181(4): 905‐913.
- Freedman B. (2015). Modelling kidney disease with iPS cells. Biomarker Insights. 10(Suppl 1): 153–169.