Toxicity Testing
With Contract Assay Services
Toxicity is a major cause of attrition in therapeutic drug development and a key consideration when selecting candidate drugs for advancement through the development pipeline. Expanding preclinical testing to incorporate assays that are better at predicting potential toxicities earlier in the development process has obvious advantages for the selection of successful lead candidates. In vitro testing on can allow investigators to preview in vivo responses, thus facilitating design of better dosing strategies and optimization of animal models in preclinical testing, as well as Phase I clinical trials.
We specialize in performing in vitro assays on to measure the potential toxic effects of candidate therapeutics, including small molecule compounds and biologics. , which generate key components of bone, fat and cartilage, may also be tested in the colony-forming unit - fibroblast (CFU-F) assay to predict possible cytotoxic effects. Mouse intestinal organoids can also be used to assess the effects of candidate therapeutics on cellular viability.
Contact us for a more in-depth discussion on how each of the assays in the sections below can be modified to meet your specific goals.
Colony-Forming Unit (CFU) Assay
We can assess the hematotoxicity of candidate therapeutics on erythroid, myeloid, and megakaryocyte progenitors using standardized and custom-designed colony-forming unit (CFU) assays (also known as the colony-forming cell (CFC) assay).
Advantages of the CFU Assay
- Yields clinically predictive information1,2, allowing for better planning and fewer in vivo studies
- Uses primary hematopoietic cells from human, non-human primate, mouse, rat or dog, to assist in selection of appropriate animal models
- Assesses both the proliferation and differentiation of erythroid (BFU-E) and myeloid (CFU-GM) progenitors


- Pessina A et al. (2003) Application of the CFU-GM Assay to Predict Acute Drug-Induced Neutropenia: An International Blind Trial to Validate a Prediction Model for the Maximum Tolerated Dose (MTD) of Myelosuppressive Xenobiotics. Toxicological sciences 75(2) 355-367.
- Pessina A et al. (2009) Application of human CFU-Mk assay to predict potential thrombocytoxicity of drugs. Toxicol In Vitro 23(1) 194-200.
HemaTox - Liquid Culture-Based Hematotoxicity Assays
Advantages of HemaTox™ Liquid Medium-based Assays
- Assesses both proliferation and differentiation of erythroid, myeloid and megataryocyte progenitors in < 10 days
- Allows high-throughput testing of compounds in 96-well format
- Exhibits correlation with the CFU assay for toxicity levels of a wide range of compounds
- Allows high flexibility as test compounds may be added to the culture at different time points, allowing the effects on progenitor cells at different stages of differentiation to be examined
- Provides improved sensitivity in the evaluation of anti-proliferative effects

Figure 2. HemaTox™ Assay Workflow

Figure 3. Correlation Between IC50 and IC90 Values for 43 Test Compounds Using the CFU-GM Assay and the Liquid-Based HemaTox™ Myeloid Assay

Figure 4. Dose-response Curves of Different 5-FU Treatment Regimens
CD34+ cells were exposed to 5-Fluorouracil continuously for the entire duration of the culture (red, continuous), transiently for 24 hours on day 0 followed by washout of the drug (black, 24-Hour treatment, day 0), and transiently for 24 hours on day 6 (blue, 24-Hour treatment, day 6), when committed myeloid progenitors were already present. Data is presented as average growth, as a percentage of control, obtained from triplicate culture wells derived from a single representative donor. Error bars represent standard deviation.
AZT Case Study
Azidothymidine (AZT) is an antiviral nucleoside analog that targets viral polymerases but can also inhibit cellular polymerases, leading to decreased cell proliferation and ultimately the suppression of hematopoiesis, resulting in anemia and neutropenia. Traditional CFU assays primarily quantitate the effects of a drug on colony numbers and not effects on colony size that may result from inhibited cell proliferation. The inability to quantify changes in colony size may explain why AZT, well known to perturb hematopoiesis in patients, does not exhibit high toxicity in CFU assays. In contrast, HemaTox™ assays can detect changes in both cell differentiation, by assessing the expression of cell surface markers used to distinguish between specific cell populations, and cell proliferation, by absolute cell counts.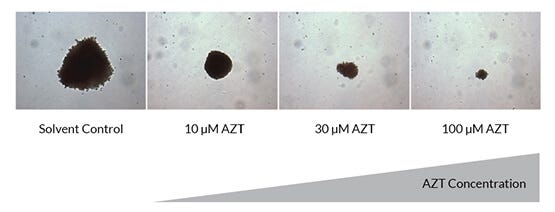
Figure 5. Strong Anti-Proliferative Effects of AZT can be Qualitatively Observed in the CFU Assay by Changes in Colony Size
Shown are erythroid (BFU-E) colonies at 10X magnification in a CFU assay after 14 days of culture in the absence and increasing presence of AZT.
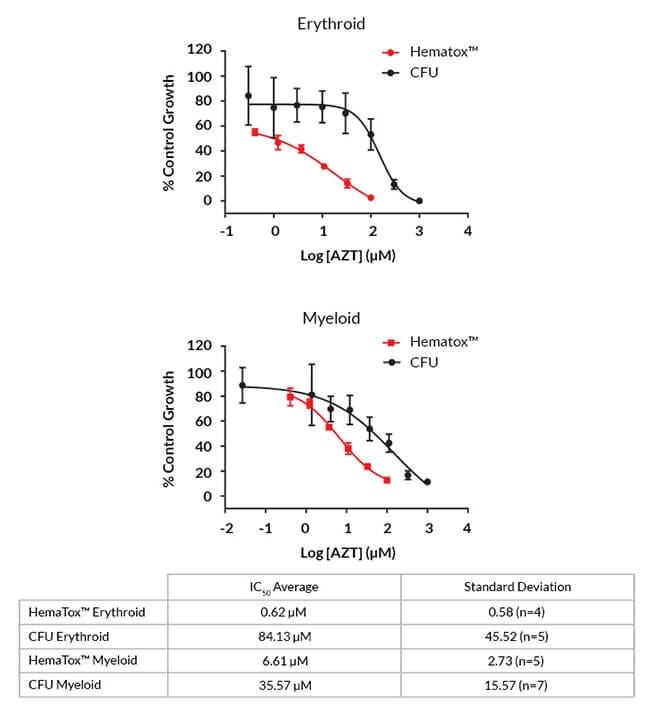
Figure 6. HemaTox™ Assays Show Greater Toxicity of AZT when Compared with CFU Assays
Representative dose-response curves for AZT based on colony numbers (black, CFU) and lineage-specific cell numbers (red, HemaTox™). The table shows the average IC50 values from three to five multiple donor lots and four to seven independent experiments.
- Provides high reproducibility with pre-qualified primary stem cells from human cord blood or bone marrow

Figure 7. Inter-Donor and Inter-Assay Reproducibility of 5-FU Dose-Response Curves
Dose-response curves were generated from titrations of 5-Fluorouracil added to human CD34+ cells from five to seven donor lots in HemaTox™ (A) Myeloid, (B) Erythroid and (C) Megakaryocyte assays. In each assay, similar IC50 values were obtained with cells from different donors and in different experiments with cells from the same donor. Shown are values (% of control growth) normalized to the number of cells in the solvent control cultures.
Read more about Services for Predicting Hematotoxicity in Drug Development with HemaTox™ Assays
Mesenchymal Toxicity Assessment
The CFU-F Assay as Performed by Contract Assay Services
- Measures the effects of a test article on progenitor frequency
- Assesses proliferative or expansion potential of progenitors (size and morphology of colonies)
- Quantitates mesenchymal progenitors in bone marrow
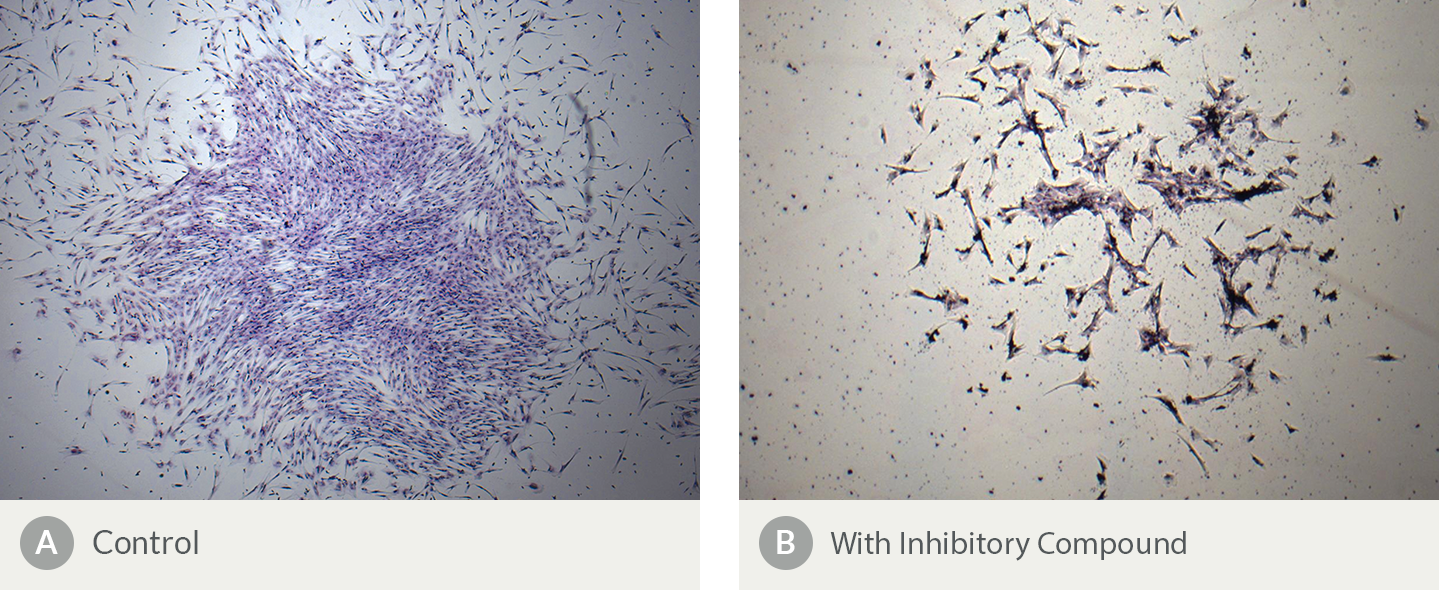
Figure 8. The Presence of an Inhibitory Compound Changes the Morphology of Human Bone Marrow-Derived CFU-F Colonies
Shown are Colony-Forming Unit - Fibroblast (CFU-F) assays containing MSCs plated (A) in the absence and (B) in the presence of an inhibitory compound. Notable differences in morphology include fewer cells and a more scattered distribution in the culture containing (B) the inhibitory compound. Colony numbers are also reduced in the presence of an inhibitory compound (data not shown).
Intestinal Organoid Models for Toxicity Assessment
The physiological relevance of cell-based assays can potentially be increased through the use of 3D culture systems, bridging the gap between high-throughput in vitro screening methods and large in vivo studies during drug development.1,2 Organoids generated from the intestinal epithelium recapitulate numerous features of the adult intestine in vivo, including self-renewal and differentiation pathways, cell types present and cellular organization within the epithelium. Together these characteristics create a culture system that is a powerful tool for investigating the potential toxicity of candidate therapeutics to the intestinal epithelium.
Contract Assay Services offers an in vitro intestinal organoid-based assay to assess the effect of candidate therapeutics on cell viability via measurement of intracellular ATP. Read more about Development of a 96-well Assay for Assessing Cell Viability in Mouse Small Intestinal-Derived Organoids After Treatment with Cytotoxic Compounds.
Learn more about organoid-based assays for intestinal toxicity testing >
- Ranga A et al. (2014) Drug discovery through stem cell-based organoid models. Adv Drug Deliv Rev 69-70 19-28.
- Sato T, Clevers H. (2013) Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340(6137) 1190-1194.


