Virtual Conference Exhibition: Drug Discovery and Toxicity Testing
Introduction
Toxicity testing is paramount when screening newly developed drugs to advance them through the development pipeline. Using the right cell-based models and assays for such testing in the early stages of your research can help reduce late-stage attrition.
STEMCELL Technologies is here to bolster your drug development and toxicity testing research. With personalized services, custom products, flexible delivery times, and the option to reserve entire lots to pre-screen cells for applications, we help you get the ethically sourced, human primary cells you need. In addition, our Contract Assay Services (CAS) specialize in primary cell-based assays and can work with you to design and perform your drug development studies.
Browse scientific presentations and resources below to learn about our human primary cells, Contract Assay Services, or other products and services for customized, flexible solutions to support your unique regulatory or product requirements.
Bookmark this page for easy reference and follow us @STEMCELLTech on Twitter for updates.
Contract Out Your In Vitro Assays
Contract Assay Services (CAS) combines the power of specialized STEMCELL products with the practical knowledge of our scientists to provide both standardized and customized assay services. Partner with our in-house contract research organization (CRO) to obtain timely, high-quality, and clinically relevant data.
Toxicity Testing
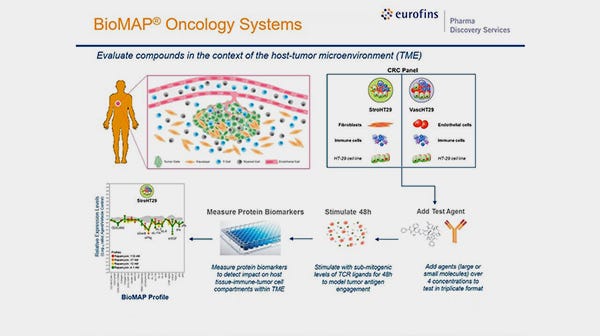
In vitro testing on primary cells allows you to preview in vivo responses, facilitating design of better dosing strategies and optimization of animal models in preclinical testing, as well as phase I clinical trials. We specialize in performing in vitro assays to assess hematotoxicity and mesenchymal toxicity to help you measure the potential toxic effects of candidate therapeutics, including small molecule compounds and biologics.
Explore Now >Immunology Services
Let us help you evaluate preclinical test compounds and biologics for their abilities to modulate the immune system. We offer customized cell-based assays based on your specific needs and can help you design studies to evaluate the effects of potential immunomodulatory agents using fresh or frozen cell samples.
Explore Now >Biopharmaceutical and Biosimilar Assessment Services
Biopharmaceutical drugs, also known as biologics, have become an essential part of modern pharmacotherapy and include examples such as biological proteins, cytokines, hormones, monoclonal antibodies, vaccines, and cell- and tissue-based therapies. Test the activity of your biopharmaceutical cytokines efficiently with our hematopoietic colony-forming cell (CFC) or colony-forming unit (CFU) assay services.
Learn More >Stem Cell-Related Services
Create efficiencies in your human pluripotent stem cell (hPSC), hematopoietic stem and progenitor cell (HSPC), or mesenchymal stem cell (MSC) research by partnering with our in-house experts and obtain timely and relevant data from a range of stem cell-related services, including functional and phenotypic assessments, characterization, banking, and more.
Learn More >Explore Custom Solutions
Customizable Services
We will work with you to develop tailor-made, flexible solutions to support your unique cell separation and cell culture needs.
Discover More Products for Your Research
If you work with human primary cells*, you know how important sourcing the right product for your research is. As part of our commitment to helping you find ethically sourced cells and workflows approved for use in your applications, we recently launched these new products.
Easy 250 EasySep™ Magnet
Scale up your manual cell isolations with the new Easy 250 EasySep™ Magnet. With this new magnet you can now process up to 225 mL and 12.5 x109 cells at once in as little as 20 minutes.
Browse our complete human primary cell portfolio* for additional new products and select from a range of cells sourced from peripheral blood, cord blood, and bone marrow. Also find answers to frequently asked questions (FAQs) about primary cells, or contact us directly for additional information.
*Certain products are only available in select territories.
**Fresh immune cell subsets are not available in Canada.
Watch Webinars
Featured Resources for Your Research
Cell Counting Resources
Access templates and resources designed to help you accurately count cells and measure cell viability.
E-Book: Cell Separation Techniques & Protocols
Download this comprehensive guide and brush up on the basic techniques of cell isolation with this ultimate guide to cell separation.
Methods Library
Master key lab techniques with this library of detailed protocols, tips and tricks, video demonstrations, and more.
Receive a Free Wallchart
Choose from a range of wallcharts covering topics related to immunology, COVID-19, stem cell biology, cancer research, and more!