Truly Model the Human Airway
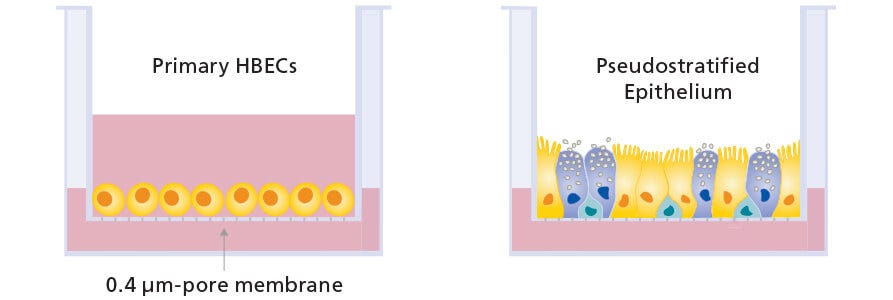
Air-liquid interface (ALI) culture of primary airway epithelial cells is increasingly recognized as important for enabling physiologically relevant respiratory research. However, obtaining truly differentiated ALI cultures can be challenging, due to the limited expansion and differentiation potential associated with primary cells. Recent studies have highlighted the need for more critical assessments of expansion and differentiation culture conditions to obtain optimal morphology and functional readouts.1
Explore our resources and comparative data below, and ask yourself whether or not your airway cultures truly model the human airway.
Studying COVID-19 with Air-Liquid Interface Culture
ALI culture of primary airway epithelial cells is a uniquely suited model system for the study of respiratory infections in vitro. Learn about the features that make ALI cultures a physiologically relevant model system, and see how researchers are using this system for infection studies of the novel coronavirus and other respiratory viruses.
The PneumaCult™ Media System to Support Human Airway Cultures
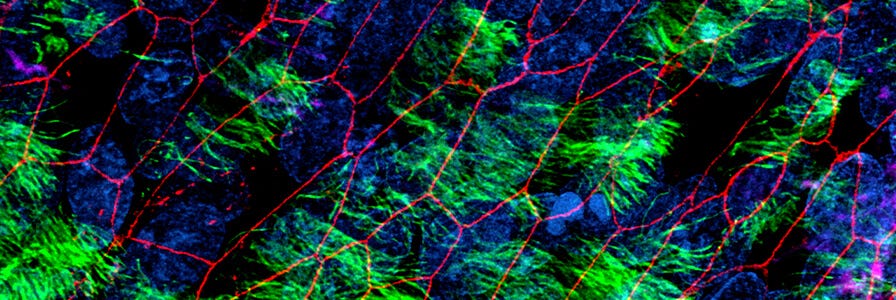
In vitro models of the human airway are instrumental in studying basic and applied aspects of respiratory biology and diseases. Cells expanded and differentiated with the PneumaCult™ system retain key features of the in vivo airway epithelium, such as a pseudostratified, mucociliated epithelial cell layer consisting of basal cells, goblet cells, and ciliated cells.
Modeling Cystic Fibrosis Airway
Studying Cystic Fibrosis Using Primary Human Nasal Epithelial Cells
References
- Rayner R et al. (2019) Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for in vitro Lung Model Studies Sci Rep 9: 500.