Immune Cell Engineering: Methods and Applications
Immunology Feature

In 2015, groundbreaking studies describing new applications of the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) led to this gene editing technology being named “Breakthrough of the Year” by Science.
The ability to genetically modify immune cells provides a powerful tool for basic and clinical researchers. The discovery of CRISPR/Cas9, which allows for more targeted gene editing1, has propelled this area of research to new heights. Here, we highlight publications describing methods for cell engineering, and showcase how immune cell engineering can be used in clinical applications, particularly for the development of cellular therapies such as chimeric antigen receptor (CAR) T cells.
Methods of Immune Cell Engineering
Scientists have been working diligently to optimize methods of immune cell engineering. For example, the following two studies describe methods to efficiently deliver gene editing nucleases into primary human immune cells.
Targeted gene knockout by direct delivery of zinc-finger nuclease proteins2
Nature Methods
The delivery of new genetic components encoding proteins or enzymes into primary immune cells is commonly achieved via viral transduction; however, there are concerns regarding undesirable side effects of viral vectors. Here, Gaj et al. sought to find an efficient method to deliver zinc-finger nucleases (ZFNs), which induce site-specific DNA double-stranded breaks to alter specific genes, without the use of viral vectors. The authors demonstrated that ZFN proteins can be directly delivered into primary human CD4+ T cells to disrupt endogenous genes using their method.
Cell separation: RosetteSep™ Human CD4+ T Cell Enrichment Cocktail
Efficient delivery of nuclease proteins for genome editing in human stem cells and primary cells3
Nature Protocol
In addition to ZFNs, transcription activator-like effector nucleases (TALENs) and CRISPR/Cas9 have also been described as effective tools to introduce gene editing. Here, Liu et al. outlined methods to efficiently deliver these site-specific DNA endonucleases into primary CD4+ T cells and human embryonic stem cells without the use of viral transduction. TALENs can be conjugated to cell-penetrating peptides to promote cellular uptake, while Cas9 can be pre-incubated with its single-guide RNA (sgRNA) and then delivered by nucleofection as outlined in this Nature Protocol.
Cell separation: EasySep™ Human CD4+ T Cell Isolation Kit, SepMate™ and mTeSR™1
Despite being powerful gene editing tools, ZFN, TALEN and CRISPR/Cas9 are not perfect. As a result, researchers are continually working to improve the specificity and efficiency of gene editing tools. For example, CRISPR-Cpf1, a class 2 CRISPR system, may help to even more precisely introduce DNA into the genome4. In addition, scientists have also engineered genome-editing technologies that can specifically convert a single target DNA base without requiring dsDNA breaks based on the CRISPR system5.
Applications of Immune Cell Engineering
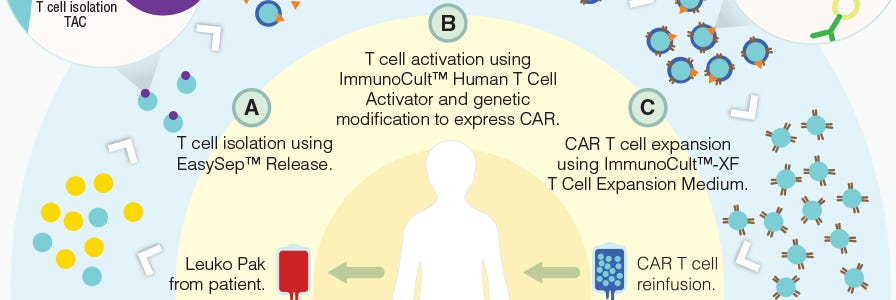
T cells can be genetically engineered to target and kill tumor cells using chimeric antigen receptors (CARs)6. With the invention of this technology, researchers are developing creative methods to improve the manufacturing of CAR T cells (Figure 1) and expand the applications of immune cell engineering for therapeutic purposes.
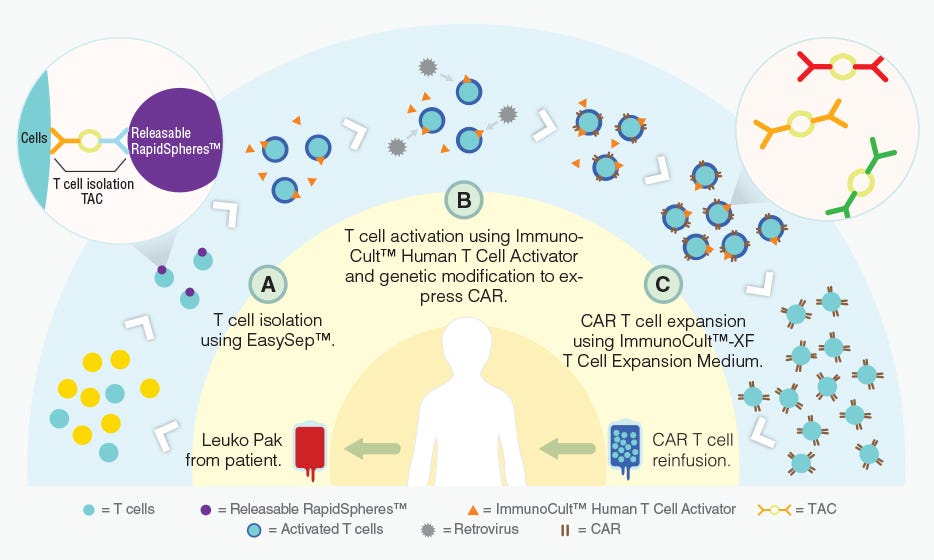
Figure 1. Proposed workflow for the manufacturing of CAR T cells using STEMCELL products.
Adapted from a Nature Dealmakers BioPharma article on innovative products for CAR T cell manufacturing.
Precision tumor recognition by T cells with combinatorial antigen-sensing circuits7
Cell
Classically, CARs are designed to target a single tumor-specific antigen; however, such specific antigens are uncommon and thus CAR T cell activation in bystander tissues expressing the same antigen can occur. Here, Roybal et al. developed a dual receptor system, where antigen A is required to induce the expression of CAR, and antigen B is required for the activation of CAR T cells (Figure 1). As a result, these engineered T cells are activated only when both antigens are present in a localized environment. This novel system not only improves the precision of tumor targeting by CAR T cells, but also expands the potential for CAR T cell use with the more common tumor-associated antigens rather than tumor-specific antigens.
Cell separation: RosetteSep™ Human CD4+ T Cell Enrichment Cocktail and RosetteSep™ Human CD8+ T Cell Enrichment Cocktail
Bacterial virulence proteins as tools to rewire kinase pathways in yeast and immune cells8
Nature Letter
Adoptive T cell therapies such as CAR T cells are being developed to treat cancer; however, there are some concerns that need to be addressed before widespread application of these therapies to humans. One particular challenge is preventing the over-activation or off-target activation of donor T cells. In this article, the authors designed a method to temporarily limit or pause T cell activity without eliminating these donor cells. Wei et al. engineered constructs that allow for the inducible expression of bacterial virulence proteins that disrupt MAPK signaling to reversibly turn off T cells. Further research aimed at designing a better “off-switch” for adoptively transferred cells will be critical in ensuring the safety of such therapies.
Cell separation: EasySep™ Human CD4+ T Cell Isolation Kit
Although the development of CAR T cells as a cellular therapy against cancer has been a primary focus in the scientific community, there are other applications for immune cell engineering. For example, regulatory T cells (Tregs) and natural killer (NK) cells can also be engineered to express CARs and can be used therapeutically to treat transplant rejection and malignancies, respectively9,10. Early clinical trials of CAR T cells in B cell malignancies have shown success11, prompting millions of dollars of investments and creating a highly competitive research landscape amongst both academic institutions and pharmaceutical companies. The coming years will reveal who comes out on top in this CAR T cell therapy race.
Explore More Immunology Features
Production of CAR T Cells Wallchart
This free Nature Protocols Wallchart summarizes the processes involved in producing CAR T cells for therapy, including cell enrichment, activation and expansion.
T Cell Therapy Reagents
Explore tools for your T cell therapy research, including products for T cell isolation, activation, and expansion.
Resources for Your Immune Cell Engineering Research
References
- Doudna JA. (2014) Science 346(6213): 1258096.
- Gaj T et al. (2012) Nat Methods 9(8): 805-807.
- Liu J et al. (2015) Nat Protoc 10(11): 1842-1859.
- Zetsche B et al. (2015) Cell 163(3): 759-771.
- Komor AC et al. (2016) Nature 533(7603): 420-404.
- Jackson HJ et al. (2016) Nat Rev Clin Oncol. 13(6): 370-383.
- Roybal KT et al. (2016) Cell 164(4): 770-779.
- Wei P et al. (2012) Nature 488(7411): 384-388.
- MacDonald et al. (2016) J Clin Invest 126(4):1413-1324.
- Glienke W et al. (2015) Front Pharmacol 6(21).
- Maude SL et al. (2015) Blood 125: 4017-4023.