Mesenchymal Stromal Cell Updates and Debates
Mesenchymal stromal cell (MSC) research has grown exponentially over the past decade and there are currently over 350 active clinical trials utilizing MSCs. Since their discovery in the 1970s by Friedenstein et al.1,2, MSCs have been referred to by many terms. Many leaders in the field claim that the most commonly used term for these cells—mesenchymal stem cells, coined by Caplan3—has been a source of confusion and exploitation. As the field continues to grow and the number of gaps in our fundamental understanding of MSCs become clearer, the need for clarification and open discussion becomes more evident.
Recent Commentaries on the State of MSC Research
... Answers will be found in better science.
Sipp et al., Nature 2018
Clear Up This Stem-Cell Mess4
In their recent Nature commentary, Drs. Sipp, Robey, and Turner call for a “coordinated global effort" to better characterize and understand the biology of MSCs, which can go by many names but are most commonly—and confusingly—referred to as “mesenchymal stem cells". MSC is currently a broad term that encompasses a variety of cell types. For about two decades, scientists and clinicians have questioned the name and biological functions associated with these cells but “the field continues to be a mess". The confusion around these cells has led to a “false magical image" of MSCs and still unproven stem cell treatments for a variety of medical conditions. To address these concerns, the authors suggest improving our understanding of MSC biology. Standardized analyses of gene expression and differentiation assays can determine the true identity of different cell types currently collectively known as MSCs. The cells that fall under current MSC description exhibit drastically variable gene expression and differentiation capacity based on their originating tissue5, suggesting that referring to them using the same term is not categorically correct. Clearing up the MSC mess will no doubt require the effort of the entire community, but Sipp et al. suggest that describing these cells by their originating tissue instead of referring to everything as mesenchymal stem cells would be a good start.
... Drop the stem cell nomenclature because it is scientifically and therapeutically misleading.
Caplan, Stem Cells Translational Medicine 2017
Mesenchymal Stem Cells: Time to Change the Name!6
In a call for a name change, Dr. Arnold Caplan, the scientist who first coined the term “mesenchymal stem cells"3, explains why he believes that this term is no longer appropriate. Dr. Caplan believes that “medicinal signaling cells" is the most relevant term for referring to MSCs. This is because studies have shown that MSCs exert their therapeutic effect by residing in sites of injury and secreting signaling factors7 with regenerative and immunomodulatory effects8,9, thus acting as therapeutic drug producers. Dr. Caplan argues that MSCs are not stem cells and that misconceptions in the field have led to unsubstantiated claims that mislead the public. In his view, a name change for MSCs is therefore necessary to reflect the fact that in many cases these cells do not behave as tissue progenitors in the body and should not be referred to as stem cells.
...This paracrine-centric viewpoint of MSC is grossly oversimplified…
Boregowda et al., Stem Cells 2018
Mesenchymal Stem Cells: The Moniker Fits the Science10
In their commentary on Dr. Caplan's 2017 perspective article6, Boregowda et al. argue that referring to MSCs as “medicinal signaling cells" oversimplifies decades of research findings. The authors believe that the newly proposed nomenclature “...describes only one salient feature of MSCs and their perceived mode of action following their nonhomologous use in human patients". While it is evident that MSC-paracrine acting factors can contribute to therapeutic potency8,11, transplanted MSCs have been shown to differentiate into pericytes and bone lining osteoblasts and osteocytes in addition to reconstituting a functional hematopoietic niche12. The authors discuss multiple other studies that demonstrate stem/progenitor properties for MSCs and share their recent observation that the stem properties and medicinal signaling function of MSCs are linked. They urge the field to not adopt “medicinal signaling cells" for MSCs as it “...deflects from the critical issues at hand, which are to gain an in-depth knowledge of MSC biology in order to exploit their inherent physiological functions…".
Key MSC Research Articles
Apoptosis in mesenchymal stromal cells induced in vivo recipient-mediated immunomodulation13
Galleu et al., 2017
In their 2017 original research article, Galleu and colleagues provide novel insight into the mechanism by which MSCs exert immunosuppressive effects. The results of clinical trials utilizing MSCs as immunomodulatory agents have yet to provide definitive proof of efficacy. In most trials, only a portion of patients affected by the same disease and treated with MSCs exhibit a therapeutic response. Using a mouse model of graft-versus-host disease (GvHD), Galleu and colleagues demonstrated that transplanted MSCs undergo apoptosis in a CD8+ T cell-dependent manner. Apoptotic MSCs are then engulfed by recipient macrophages, inducing the production of indoleamine 2, 3-dioxygenase (IDO), which induces the anergy of effector T cells and ameliorates GvHD. MSC-induced immunomodulation was not observed in GvHD mouse models when cytotoxic activity of CD8+ T cells was abrogated by knocking-out Perforin or recipient macrophage depletion. Based on these results, the authors demonstrated a positive correlation between the level of patient cytotoxic response against MSCs in vitro and the therapeutic efficacy of transplanted MSCs. Galleu et al. suggest that the next generation of MSC cell therapies should focus on infusion of apoptotic MSCs or screening for patients with efficient cytotoxic response to MSCs.
Identification of the Human Skeletal Stem Cell14
Chan et al., 2018
Historically, MSCs have been known as a highly diverse cell population. Even when isolated from the same donor and/or tissue, MSCs may display significant heterogeneity in their properties and functions. In their 2018 publication, Chan and colleagues shone a light on MSC heterogeneity when they isolated a self-renewing multipotent human skeletal stem cell (hSSC). This discovery indicated that the cells currently referred to as "mesenchymal stem cells" are composed of subpopulations with distinct cell surface markers and differentiation capacities. The hSSCs described in this study are capable of generating progenitors for bone, cartilage, and stroma, but not fat; suggesting that other cell populations might be responsible for adipose tissue regeneration. hSSCs can be isolated from fetal and adult bones, BMP2-treated adipose stroma, and induced pluripotent (iPS) cells. They are marked as PDPN+CD146-CD73+CD164+, share similar characteristics when isolated from different sources, and can serve as a niche for hematopoietic stem cells. Chan and colleagues also found the presence of skeletal stem cells in mice (mSSCs), although they suggest that hSSCs and mSSCs have different intrinsic gene regulations that cause the skeletal differences observed between the two species. This recent discovery of a distinct subpopulation within the cells currently known under the broad umbrella of "MSCs" underscores the gaps in our knowledge and importance of continuing fundamental biology research in this field.
Featured Products and Resources
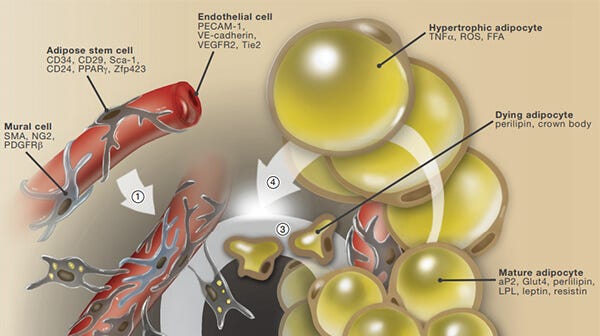
A Snapshot on the Adipocyte Life Cycle
An overview of adipose stem cell proliferation, adipocyte differentiation, turnover and obesigenic expansion.
Isolation of MSCs from Mouse Compact Bone
This instructional video provides step-by-step instructions on how to isolate MSCs from mouse compact bone.
References
- Friedenstein A & Kuralesova AI. (1971) Transplantation 12(2): 99–108.
- Friedenstein AJ et al. (1968) Transplantation 6(2): 230–47.
- Caplan AI. (1991) J Orthop Res 9(5): 641–50.
- Sipp D et al. (2018) Nature 561(7724): 455–457.
- Sacchetti B et al. (2016) Stem cell reports 6(6): 897–913.
- Caplan AI. (2017) Stem Cells Transl Med 6(6): 1445–1451.
- Meirelles L da S et al. Cytokine Growth Factor Rev 20(5–6): 419–27.
- Caplan AI & Correa D. (2011) Cell Stem Cell 9(1): 11–5.
- Caplan AI & Dennis JE. (2006) J Cell Biochem 98(5): 1076–84.
- Boregowda S V et al. (2018) Stem Cells 36(1): 7–10.
- Phinney DG & Pittenger MF. (2017) Stem Cells 35(4): 851–858.
- Muguruma Y et al. (2006) Blood 107(5): 1878–87.
- Galleu A et al. (2017) Sci Transl Med 9(416).
- Chan CKF et al. (2018) Cell 175(1): 43–56.e21.