StemSpan™ Media and Supplements for the Expansion and Differentiation of Erythroid Progenitor Cells
- Document # 27014
- Version 1.0.0
- Feb 2016
Background
Culture methods for generating erythroid cells from hematopoietic stem and progenitor cells (HSPCs) isolated from human umbilical cord blood (CB), bone marrow (BM) or peripheral blood (PB) are important for the study of erythropoiesis. This includes research into the regulation of normal and aberrant erythropoiesis, and other applications, such as the generation of somatic target cells for reprogramming into induced pluripotent stem (iPS) cells.
Media and Supplements for the Expansion and Erythroid Differentiation of Human HSPCs
StemSpan™ Serum-Free Expansion Media, SFEM () and SFEM II (), have been developed for the culture of human HSPCs. When used in combination with the StemSpan™ Erythroid Expansion Supplement (), which consists of an optimized combination of recombinant human cytokines (SCF, IL-3 and EPO), these media selectively promote the expansion and differentiation of erythroid progenitor cells in cultures of purified CD34+ cells from human CB, BM and PB. This results in very pure populations of transferrin receptor (CD71) and Glycophorin A (CD235, GlyA) positive erythroblasts after 7 - 14 days of culture (Figure 1). Cell yields are typically higher in StemSpan™ SFEM II than in SFEM; however, both media support the generation of thousands of erythroblasts per original CD34+ cell after 7 - 14 days of culture. Results for 14-day cultures of CB-derived CD34+ cells in StemSpan™ SFEM and SFEM II are shown in Figure 2 and Table 1.
StemSpan™ Erythroid Expansion Medium (ACF-E) (Catalog #09955) is a new animal component-free (ACF) medium that has been specifically developed for the expansion and erythroid differentiation of human CD34+ cells, when combined with the StemSpan™ Erythroid Expansion Supplement. StemSpan™ ACF-E is recommended for applications where the use of a medium devoid of animal- and human-derived components is required. The yield of erythroid cells in StemSpan™ ACF-E medium is significantly higher than in StemSpan™ SFEM, and similar to StemSpan™ SFEM II (Figure 2A, Table 1). The frequency of erythroid cells obtained in StemSpan™ ACF-E are similar to those obtained in StemSpan™ SFEM and SFEM II (Figure 2B and Table 1).
Advantages
- Media available in serum-free and animal component-free formulations.
- Produce thousands of erythroblasts per input CD34+ cell.
- Culture-expanded erythroblasts may be matured further or used for specific applications, such as reprogramming into iPS cells.
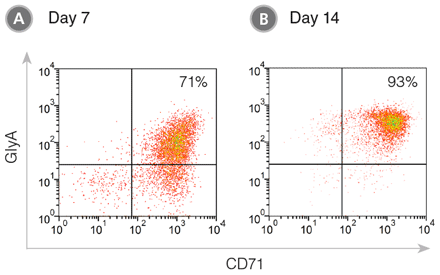
Figure 1. CD71+ GlyA+ Cells Produced from CB-Derived CD34+ Cells During 7 - 14 Days of Culture in StemSpan™ SFEM II Medium with Erythroid Expansion Supplement
CB-derived CD34+ cells were cultured in StemSpan™ SFEM II containing Erythroid Expansion Supplement. After (A) 7 days and (B) 14 days, the cultured cells were stained with CD71 () and GlyA () antibodies and analyzed by flow cytometry. (A) During differentiation, the CD34+ cells progress to the CD71+ GlyA+ erythroblast stage via CD71+ GlyA- progenitors and pro-erythroblasts, which are still abundant on day 7. (B) With further culture these cells differentiate into erythroblasts and by day 14 most cells have become CD71+ GlyA+ . A small population of cells may mature further in these cultures into CD71-/loGlyA+ normoblasts and reticulocytes.

StemSpan™ SFEM II and StemSpan™ Erythroid Expansion Supplement
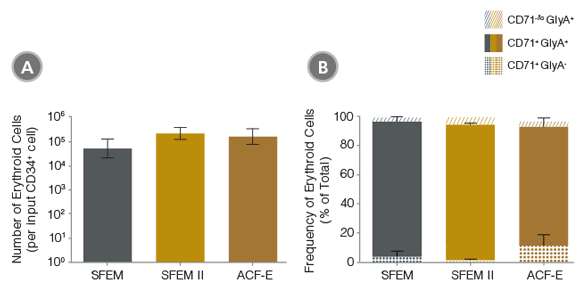
Figure 2. Production of Erythroid Cells from Human CB-Derived CD34+ Cells Cultured in StemSpan™ Media Containing StemSpan™ Erythroid Expansion Supplement
(A) Average numbers of erythroid cells generated after culturing purified CD34+ CB cells (n=15) for 14 days in StemSpan™ SFEM (gray bars), SFEM II (gold bars) or ACF-E (brown bars) media containing Erythroid Expansion Supplement. Shown are the number of erythroid cells that express CD71 and/or GlyA per input CD34+ cell. (B) The percentages of the different erythroid cell subsets generated in these cultures are shown, including CD71-/loGlyA+ normoblasts, CD71+ GlyA+ erythroblasts, and immature CD71+ GlyA- erythroid progenitors and pro-erythroblasts. Vertical lines indicate 95% confidence limits (CL). Erythroid cell yields were significantly higher in SFEM II and ACF-E than in SFEM (p < 0.05; paired t-test, n=15). See Table 1 for values and 95% CL.
Table 1. Production of Erythroid Cells from Human CB-Derived CD34+ Cells Cultured in StemSpan™ Media Containing StemSpan™ Erythroid Expansion Supplement
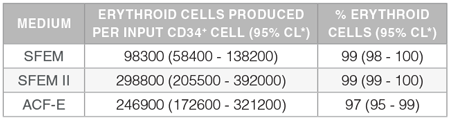
Yields and percentages of erythroid cells produced after culturing purified CD34+ CB cells (n=15) for 14 days in StemSpan™ SFEM, SFEM II or ACF-E media containing Erythroid Expansion Supplement. Erythroid cells were identified by flow cytometry after staining with antibodies against CD71 and GlyA. The % erythroid cells represent the percentage of cells that express CD71 and/or GlyA.
*CL: confidence limits.
General Method for Producing Erythroid Cells in Culture Using StemSpan™ Medium and Erythroid Expansion Supplement
The following protocol is recommended for the expansion and erythroid differentiation of human CB-derived CD34+ cells. Cell yields may reach several thousand erythroblasts per input CD34+ cell in 7 - 14 days; however, this number may vary between experiments depending on the source and quality of the starting cell population. This protocol may also be used with mononuclear cells (MNCs) isolated from PB, or CD34+ cells isolated from other tissues such as BM, though cell yields may be lower than in cultures initiated with CB-derived CD34+ cells. Further protocol optimization (e.g., testing of different plating densities, culture periods, feeding and replating schedules) may be required, depending on the specific objectives of the experiment.
With this protocol, typically more than 80% of cells generated after 14 days of culture are CD71+GlyA+ erythroblasts. However, immature CD71+GlyA- erythroid progenitors and pro-erythroblasts, and CD71-/loGlyA+ normoblasts may also be present. If desired, cultured erythroblasts may be further differentiated into CD71-/loGlyA+ normoblasts, reticulocytes and red blood cells by replating cells in medium that contains EPO (Catalog #02625) as a single growth factor. Refer to the section on Erythroid Progenitor Cell Maturation for further details (Page 3).
Protocol
- Isolate CD34+ cells from CB, BM or PB:
- To isolate CD34+ cells from whole CB use either the EasySep™ Human Cord Blood CD34 Positive Selection Kit II (Catalog #17986) or Kit III (Catalog #17987)
- To isolate CD34+ cells from MNCs derived from previously frozen CB, BM and mobilized PB, or fresh BM and mobilized PB, use the EasySep™ Human CD34 Positive Selection Kit ()
- To isolate CD34+ cells from fresh (non-mobilized) PB or buffy coat cells use the Complete Kit for Human Whole Blood CD34+ Cells (Catalog #15086)
- Prepare complete medium by supplementing StemSpan™ SFEM, SFEM II or ACF-E medium with Erythroid Expansion Supplement. The supplement is 100X concentrated and must be diluted 1 in 100 in StemSpan™ medium before use. Aliquot the volume of supplemented medium needed for plating cells and warm to 37°C before use.
- Plate the purified cells at a concentration of 1 x 104 CD34+ cells/mL. This density works well for most CB samples as it reduces the likelihood of overgrowth and loss of viability within the culture schedule described in this protocol. Higher plating densities are possible, but more frequent media changes and replating will likely be required to maintain cell viability and optimal growth.
- Culture the cells in a humidified incubator at 37°C with 5% CO2. After 3 - 4 days, feed cultures by adding an equal volume of fresh supplemented medium to the starting volume used in each culture well, i.e. doubling the total culture volume.
- After 7 days of culture, and again after 10 - 11 days, perform a complete medium change. Harvest cells from the culture and centrifuge for 5 - 10 minutes at 200 x g. Gently resuspend the cell pellet in fresh supplemented medium to bring the sample to a concentration of < 1 x 105 cells/mL.
- After 14 days, harvest the cells by centrifuging for 5 - 10 minutes at 200 x g. If desired, cells may be cultured beyond 14 days with periodic medium changes (i.e., every 3 - 4 days) and passaging to prevent overgrowth and nutrient depletion.
- Count total and viable cell numbers using a hemacytometer or automated cell counter. Assess erythroid differentiation of the cells by flow cytometry using appropriate markers (e.g., CD71 and GlyA).
General Method for Erythroid Progenitor Cell Maturation
The StemSpan™ Erythroid Expansion Supplement is primarily designed to stimulate the production of large numbers of erythroblasts by selectively promoting the proliferation and erythroid differentiation of HSPCs. Most culture-expanded cells remain CD71+GlyA+ and do not express hemoglobin (Hb). To promote the hemoglobinization and maturation of culture-expanded erythroblasts into normoblasts and reticulocytes, it is necessary to remove the Erythroid Expansion Supplement and culture the differentiated cells in medium in which EPO is the only cytokine. One suggested protocol is described below, but different approaches, which may involve serum-free media, co-culture with stromal cells or the use of compounds that block glucocorticoid receptor (GR) activity, have also been described.1-8
Protocol
- After expanding cells for 7 - 14 days in the conditions described above, collect the cells by centrifugation for 5 - 10 minutes at 200 x g, and wash once in Iscove’s MDM or in StemSpan™ SFEM or SFEM II medium without added growth factors to remove the Erythroid Expansion Supplement and any other additives.
- Resuspend the cells at a concentration of 5 - 10 x 105 per mL in fresh StemSpan™ SFEM or SFEM II medium supplemented with 3% normal human AB serum and recombinant human EPO (at a concentration of 1 - 3 U/mL). The inclusion of human serum is important to maintain cell viability. The use of StemSpan™ ACF-E medium during the maturation step is not recommended as it does not support erythroblast maturation to the same degree as SFEM and SFEM II.
- After 4 - 7 days of culture, examine the cells for CD71 and GlyA expression by flow cytometry and, optionally, for hemoglobinization by cytochemical staining of cytocentrifuged cell preparations using benzidine or a similar reagent.
During maturation, the number of cells in culture is not likely to increase significantly. As the cells mature, their size decreases as indicated by lower forward light scatter, CD71 expression declines, but remains detectable on most cells, and GlyA expression remains high. Additionally, Hb expression gradually increases and the frequency of Hb+ cells will typically peak by approximately day 7, causing cell pellets to become visibly red. Enucleation may be detectable under a microscope, indicated by the presence of reticulocytes and pyrenocytes (the extruded nucleus encased in a thin cytoplasmic layer) in culture (Figure 3). Additional markers may be used to monitor changes in antigen expression and quantify cell subsets during erythroid differentiation.9
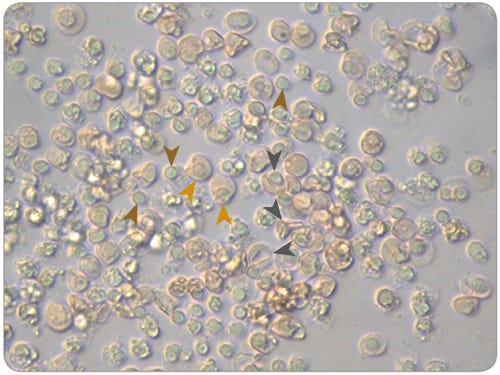
Figure 3. Morphology of Normoblasts, Reticulocytes and Pyrenocytes in Maturation Cultures of Erythroid Progenitor Cells Cultured in SFEM II
Erythroid cells (Pyrenocytes = brown arrows; Normoblasts = gold arrows; Reticulocytes = dark grey arrows) viewed under a microscope at 40X magnification after 14 days of culture in expansion conditions and 7 days of culture in maturation conditions, using SFEM II and the protocols described in this document.
Media and Supplements for Erythroid Cell Expansion
Generating Induced Pluripotent Stem Cells From Culture-Expanded Erythroblasts
PB is a popular tissue source for reprogramming somatic cells to an iPS cell state by transient overexpression of key reprogramming factors.10 Erythroid progenitor cells are an attractive starting population as they lack genomic rearrangements and can be efficiently reprogrammed.11,12 Erythroid progenitors are present in PB, but at a very low frequency (< 7 x 103 cells/mL of whole blood) and typically require expansion to obtain a sufficient number of cells for reprogramming. As the output of erythroid cells per starting progenitor cell is typically high in culture, CD34+ cell isolation is not necessary, but depletion of mature cells including RBCs and lymphocytes is recommended.
The Erythroid Progenitor Reprogramming Kit () is an integrated set of tools and reagents to enrich, expand and reprogram erythroid progenitor cells from as little as 10 mL of PB.
Erythroid Progenitor Reprogramming Workflow
- Rapidly deplete mature blood cells from PB using RosetteSep™*, Lymphoprep™* and SepMate™-15*.
- Expand erythroid cells in StemSpan™ SFEM II* (or ACF-E when ACF culture conditions are required) containing StemSpan™ Erythroid Expansion Supplement*.
- Reprogram blood cells in a feeder-free environment with ReproTeSR™*.
- Maintain iPS cells with any TeSR™ family maintenance medium, i.e., mTeSR™1 (), TeSR™2 () or TeSR™-E8™ ().
- Direct differentiation to cells of desired endodermal, mesodermal or ectodermal lineages with STEMdiff™.
For more information about the Erythroid Progenitor Reprogramming Kit, refer to the Technical Manual: Integrated Workflow for the Isolation, Expansion, and Reprogramming of Erythroid Progenitor Cells (Document #29795) available on our website at www.stemcell.com.
*Products included in the Erythroid Progenitor Reprogramming Kit.
Accessory Products
References
- Giarratana MC et al. (2005) Nat Biotechnol 23: 69-74.
- Leberbauer C et al. (2005) Blood 105(1): 85-94.
- Miharada K et al. (2006) Nat Biotechnol 24(10): 1255-6.
- Migliaccio G et al. (2010) Cell Transplant 19(4): 453-69.
- van den Akker E et al. (2010) Haematologica 95(9): 1594-8.
- Timmins NE et al. (2011) Tissue Eng Part C Methods 17(11): 1131-7.
- Giarratana MC et al. (2011) Blood 118(19): 5071-9.
- Tirelli V et al. (2011) Stem Cells Int Epub, DOI: 10.4061/2011/602483.
- Hu J et al. (2013) Blood 121(16): 3246-53.
- Zhang X-B et al. (2013) Genomics Proteomics Bioinformatics 11(5): 264–74.
- Mack AA et al. (2011) PLoS One 6(11):e27956 Epub, DOI: 10.1371/journal.pone.0027956.
- Chou B-K et al. (2011) Cell Res 21(3): 518–29.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration

