STEMdiff™ Megakaryocyte Kit
For differentiation of human ES or iPS cells to megakaryocytes and platelets
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
-
 mTeSR™ Plus
mTeSR™ PluscGMP, stabilized feeder-free maintenance medium for human ES and iPS cells
-
 DMEM/F-12 with 15 mM HEPES
DMEM/F-12 with 15 mM HEPESDulbecco's Modified Eagle's Medium/Nutrient Ham's Mixture F-12 (DMEM/F-12) with 15 mM HEPES buffer
-
 Anti-Human CD41 Antibody, Clone HIP8
Anti-Human CD41 Antibody, Clone HIP8Mouse monoclonal IgG1 antibody against human, rhesus, cynomolgus CD41
-
Labeling Antibodies
Compatible antibodies for purity assessment of isolated cells
Overview
During the first 12 days, STEMdiff™ Hematopoietic Supplement A and then Supplement MK1 are added to basal medium to induce cells toward megakaryocyte-biased hematopoietic progenitor cells. At the end of this stage, hematopoietic progenitor cells are easily harvested from the culture supernatant, and are further differentiated to mature megakaryocytes using Supplement MK2 and StemSpan™ SFEM II for an additional 5 days.
At the end of the protocol (Day 17), the cells are typically expanded 405-fold +/- 54-fold (95% CI), and on average 71% +/- 3.3% (95 CI) of cells co-express CD41a and CD42b.
STEMdiff™ Megakaryocyte Kit has been optimized for differentiation of ES/iPS cells maintained in mTeSR™1 (Catalog #85850), mTeSR™ Plus (Catalog #100-0276), or TeSR™-AOF (Catalog #100-0401).
Data Figures

Figure 1. Megakaryocyte Differentiation Protocol
The 17-day protocol includes two main stages: a 12-day stage to differentiate human embryonic stem (hES) or induced pluripotent stem (hiPS) cells into megakaryocyte-biased hematopoietic progenitor cells (HPCs), and a 5-day stage to further differentiate hES or hiPS cell-derived HPCs into mature megakaryocytes (MKs). On Day -1, hES or hiPS cells are plated as aggregates (100 - 200 μm diameter, ~100 cells per aggregate) at a density of 10 ‑ 20 aggregates/cm2 in mTeSR™1, mTeSR™ Plus, or TeSR™-AOF on Corning® Matrigel®-coated plates. After attaching overnight and confirming the number of adhered colonies is within 4 - 10 colonies/cm2, mesoderm induction is initiated by replacing TeSR™ medium with Medium A (STEMdiff™ Hematopoietic Basal Medium + STEMdiff™ Hematopoietic Supplement A). On Day 3, the medium is changed to Medium MK1 (STEMdiff™ Hematopoietic Basal Medium + STEMdiff™ Megakaryocyte Supplement MK1) for endothelial-to-hematopoietic transition (EHT) and hematopoietic specification. During this phase, hES or hiPS cell-derived HPCs emerge from an adherent layer of endothelial cells and are released into suspension. On Day 12, HPCs in suspension are harvested and replated in Medium MK2 (StemSpan™ SFEM II + STEMdiff™ Megakaryocyte Supplement MK2) at a density of 1 - 3.5 x 105 cells/mL and cultured for 5 days to generate mature MKs.

Figure 2. hPSCs Differentiate to Megakaryocyte-Erythroid Progenitors During 12 Days of Culture
hES and hiPS cells were induced to differentiate to megakaryocyte-erythroid biased HPCs following the protocol described in Figure 1. On Day 12, cells were harvested from the supernatant and analyzed for expression of CD41a, CD42b, CD34, and GlyA by flow cytometry. Dead cells were excluded by light scatter profile and propidium iodide (PI) staining. (A) Representative flow cytometry plots for hES-derived (H9) cells on Day 12. The cells show high levels of CD41a and CD42b as well as of CD34 and GlyA expression, indicating that the protocol supports differentiation of hPSCs to megakaryocyte-erythroid progenitors by Day 12. (B) Frequencies and numbers of CD41a+CD42b+ cells per input cell for two hES cell lines (H1 and H9) and two hiPS cell lines (WLS-1C and STiPS-R038). The average frequency of viable CD41a+CD42b+ cells on Day 12 ranged between 33% and 62%. The average yield of CD41a+CD42b+ cells generated per input cell ranged between 72 and 174. Data are shown as mean ± SEM (n = 7 for H1, n = 20 for H9, n = 19 for WLS-1C, n = 7 for STiPS-R038).

Figure 3. hPSC-Derived HPCs Efficiently Expand and Differentiate to CD41a+CD42b+ Megakaryocytes During an Additional 5 Days of Culture
hPSC-derived HPCs on Day 12 were cultured for 5 additional days in Medium MK2 to promote differentiation into mature MKs following the protocol described in Figure 1. Cells were harvested and analyzed for expression of CD41a, CD42b, CD34, and GlyA by flow cytometry. Dead cells were excluded by light scatter profile and PI staining. (A) Representative flow cytometry plots for hES-derived (H9) cells on Day 17. The cells showed high levels of CD41a and CD42b and low levels of GlyA and CD34 markers, indicating differentiation to megakaryocytes. (B) Frequencies and numbers of CD41a+CD42b+ MKs per input cell for two hES cell lines (H1 and H9) and two hiPS cell lines (WLS-1C and STiPS-R038). The average frequency of viable CD41a+CD42b+ cells on day 17 ranged between 56% and 77%. The average yield of CD41a+CD42b+ MKs generated per input cell ranged between 223 and 425. Data are shown as mean ± SEM (n = 12 for H1, n = 29 for H9, n = 27 for WLS-1C, n = 12 for STiPS-R038).

Figure 4. hPSC-Derived Megakaryocytes Generated Using STEMdiff™ Megakaryocyte Kit Are Polyploid
hPSC-derived MKs obtained using STEMdiff™ Megakaryocyte Kit display mature and adult-like features: cellular enlargement and polyploidization. (A) A representative bright-field image taken on Day 17 showing large MKs derived from H1 cells (10X magnification). (B) Representative immunofluorescence images taken on Day 17 showing that CD41a+ MKs derived from H1 and WLS-1C cells are polyploid (20X and 63X magnification, respectively). The cells were formaldehyde-fixed and stained with a fluorescein-conjugated antibody against surface marker CD41a (green), and DAPI (blue). (C) A representative cytospin of MKs derived from H9 cells on Day 17 showing high ploidy (40X magnification, May-Grunwald Giemsa stain). (D) Representative flow cytometry histogram and scatter plot showing the DNA ploidy profile of ethanol-fixed MKs derived from H9 cells on Day 17. The DNA content was determined by the quantity of PI staining, with different peaks on the histogram representing 2N, 4N, and 8N+ cells. Ploidy analysis was done on gated CD41a+ cells. (E) Ploidy distribution of MKs generated from two hES cell lines (H1 and H9) and two hiPS cell lines (WLS-1C and STiPS-R038). The average ploidy distributions of CD41a+ cells on Day 17 were 66%, 24.5%, and 9.5% for 2N, 4N, and 8N+, respectively. Data are shown as mean ± SEM (n = 6 for H1, n = 28 for H9, n = 19 for WLS-1C, n = 10 for STiPS-R038).
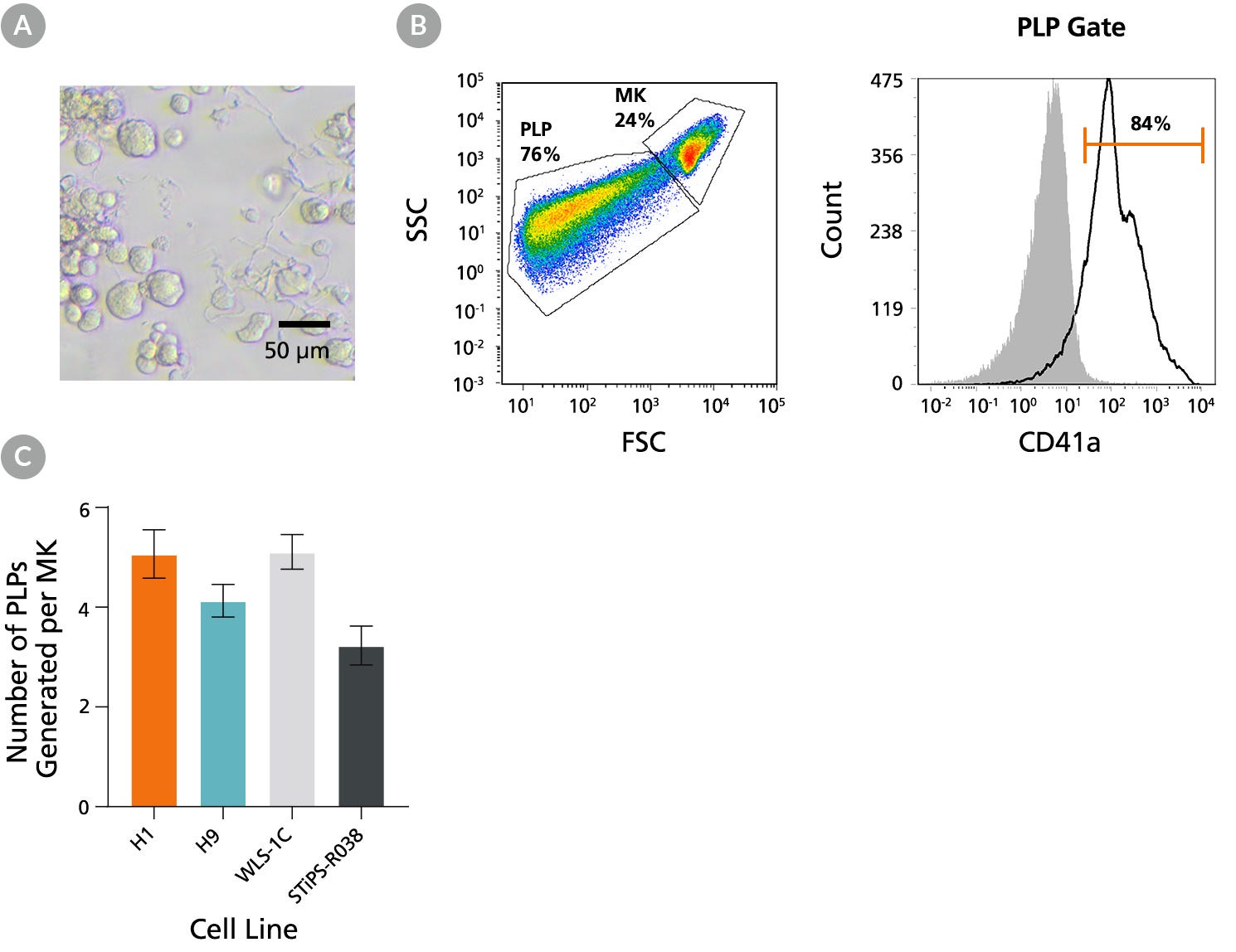
Figure 5. hPSC-Derived Megakaryocytes Generated Using STEMdiff™ Megakaryocyte Kit Yield Platelet-Like Particles
hPSC-derived MKs obtained using STEMdiff™ Megakaryocyte Kit are capable of proplatelet formation to yield functional platelet-like particles (PLPs). (A) A representative bright-field image taken on Day 17 showing MKs derived from H1 cells formed proplatelets, (long cytoplasmic protrusions,10X magnification). (B) Representative flow cytometry forward/side scatter profile of MKs and PLPs and histogram of PLPs derived from H9 cells on Day 17. The PLP gate is based on control platelets (PLTs) prepared from fresh blood. Cells were also stained with antibodies against CD41a, CD45, and GlyA for PLP characterization and enumeration. PLPs showed a high level of CD41a expression (and no CD45 and GlyA expression, data not shown) as in control PLTs. Grey filled histogram represents CD41a Fluorescence Minus One (FMO) control. (C) Numbers of PLPs generated per MK on Day 17 for two hES cell lines (H1 and H9) and two hiPS cell lines (WLS-1C and STiPS-R038). PLPs and MKs were enumerated based on the number of CD41a+CD45-GlyA- cells in the PLP gate and viable CD41a+ cells in the MK gate, respectively. The average yield of PLPs generated per MK ranged between 3.2 and 5.1. Data are shown as mean ± SEM (n = 12 for H1, n = 28 for H9, n = 27 for WLS-1C, n = 12 for STiPS-R038).

Figure 6. STEMdiff™ Megakaryocyte Kit Produces More Megakaryocytes and Platelet-Like Particles than Other Published Protocols
hES and hiPS cells were differentiated into MKs using STEMdiff™ Megakaryocyte Kit and using four different published protocols from the literature with modifications. (A) Frequencies and numbers of CD41a+CD42b+ MKs per input cell for two hES cell lines (H1 and H9) and two hiPS cell lines (WLS-1C and STiPS-R038) were analyzed by flow cytometry as shown in Figure 2. (B) Numbers of PLPs generated per input cell were enumerated as described in Figure 5. Compared to the published protocols, STEMdiff™ Megakaryocyte Kit produced 10- to 40-fold more CD41a+CD42b+ MKs and 6- to 23-fold more PLPs per input cell. P values were calculated using a one-way ANOVA followed by Dunnett’s post hoc test (*p < 0.05, **p < 0.01). Data are shown as mean ± SEM (n = 7 - 8).
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (7)
Related Products
-
 MethoCult™ SF H4636
MethoCult™ SF H4636Serum-free methylcellulose-based medium with recombinant cytokines for human ES and iPS cell-derived cells
-
 STEMdiff™ Hematopoietic Kit
STEMdiff™ Hematopoietic KitFor differentiation of human ES or iPS cells into hematopoietic progenitor cells
-
 STEMdiff™ Erythroid Kit
STEMdiff™ Erythroid KitFor differentiation of human ES or iPS cells to erythroblasts
-
 mTeSR™1
mTeSR™1cGMP, feeder-free maintenance medium for human ES and iPS cells
Item added to your cart

STEMdiff™ Megakaryocyte Kit
Quality Statement:
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.








